Mangrove-Derived Fungi as a Reservoir of Bioactive Secondary Metabolites Promising for Anticancer Leads: A Literature Review
DOI:
https://doi.org/10.36733/medicamento.v9i2.6910Keywords:
anticancer, cytotoxic, mangrove-derived fungi, secondary metabolitesAbstract
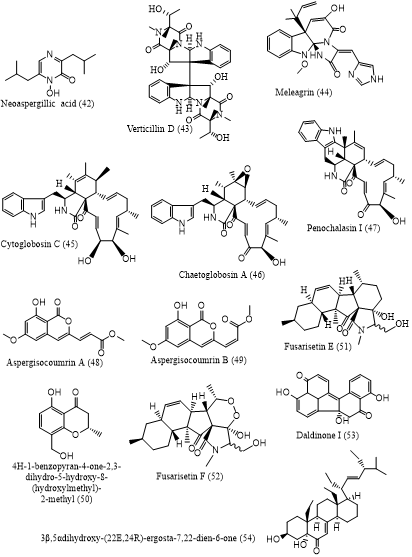
Cancer was the leading cause of death, which accounting for nearly 10 million deaths globally in 2020. Currently, cancer treatment still relies on chemotherapy, however, most anticancer drugs showed non-specific toxicity to normal cell proliferation resulting in various side effects, and are ineffective against many forms of cancer. In addition, the increasing case of chemoresistance of cancer cells to chemotherapy has boosted the discovery of new anticancer agents. Natural products are known as the origin of several clinically used anticancer agents, e.g. taxol and vincristine. Among natural products, mangrove-derived fungi are of particular scientific interest evidenced by the increasing rate of publications on cytotoxic secondary metabolites reported. Hence, this literature review aims to provide comprehensive information on cytotoxic secondary metabolites isolated from mangrove-derived fungi, which might contribute to the search for anticancer leads from natural resources. Data were collected from original research articles published on scientific-based sources such as Google Scholar, PubMed, Taylor and Francis, Elsevier, and MDPI, in the range of 2011-2022. Fifty-four cytotoxic secondary metabolites with IC50 values below 10 µM were described herein, which were classified in to 8 groups of metabolites. These compounds were reported from 16 genera of mangrove-associated fungi. Among them, Aspergillus and Penicillium were the most frequent producers of cytotoxic metabolites, suggesting their enormous potential as a source of pharmacophores for anticancer candidates.
References
Li KH, Griffin T, Nikbakht N, et al. Neoplasms. Pract Immunodermatology. Published online 2016:279-296. doi:10.1007/978-94-024-0902-4_12
Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209-249. doi:10.3322/caac.21660
WHO. WHO Report On Cancer In Indonesia.; 2020. WHO, CANCER REPORT 2020
Joel EL, Valentin Bhimba B. Evaluation of Secondary Metabolites from Mangrove Associated Fungi Meyerozyma guilliermondii. Alexandria J Med. 2013;49(3):189-194. doi:10.1016/j.ajme.2013.04.003
Ali Abdalla YO, Subramaniam B, Nyamathulla S, et al. Natural Products for Cancer Therapy: A Review of Their Mechanism of Actions and Toxicity in the Past Decade. J Trop Med. 2022;2022. doi:10.1155/2022/5794350
Ariantari NP, Ancheeva E, Frank M, et al. Didymellanosine, a New Decahydrofluorene Analogue, and Ascolactone C from: Didymella sp. IEA-3B.1, an Endophyte of Terminalia catappa. RSC Adv. 2020;10(12):7232-7240. doi:10.1039/c9ra10685e
Handayani D, Mulia P, Andayani R, Wahyuni FS, Ariantari NP. Secondary Metabolite from Mangrove Endophytic Fungus Fusarium proliferatum AED3. Rasayan J Chem. 2021;2021(Special Issue):150-155. doi:10.31788/RJC.2021.1456447
Pudhom K, Teerawatananond T, Chookpaiboon S. Spirobisnaphthalenes from the Mangrove-Derived Fungus Rhytidhysteron sp. AS21B. Mar Drugs. 2014;12(3):1271-1280. doi:10.3390/md12031271
Shearer CA, Descals E, Kohlmeyer B, et al. Fungal Biodiversity in Aquatic Habitats. Biodivers Conserv. 2007;16(1):49-67. doi:10.1007/s10531-006-9120-z
De Souza Sebastianes FL, Romão-Dumaresq AS, Lacava PT, et al. Species Diversity of Culturable Endophytic Fungi from Brazilian Mangrove Forests. Curr Genet. 2013;59(3):153-166. doi:10.1007/s00294-013-0396-8
Dulo B, Phan K, Githaiga J, Raes K, De Meester S. Natural Quinone Dyes: A Review on Structure, Extraction Techniques, Analysis and Application Potential. Vol 12. Springer Netherlands; 2021. doi:10.1007/s12649-021-01443-9
Chen H, Zhu X, Zhong LL, et al. Synthesis and Antitumor Activities of Derivatives of the Marine Mangrove Fungal Metabolite Deoxybostrycin. Mar Drugs. 2012;10(12):2715-2728. doi:10.3390/md10122715
Mishra PD, Verekar SA, Deshmukh SK, Joshi KS, Fiebig HH, Kelter G. Altersolanol A: A Selective Cytotoxic Anthraquinone from a Phomopsis sp. Lett Appl Microbiol. 2015;60(4):387-391. doi:10.1111/lam.12384
Isaka M, Chinthanom P, Rachtawee P, et al. Cytotoxic Hydroanthraquinones from the Mangrove-Derived Fungus Paradictyoarthrinium diffractum BCC 8704. J Antibiot (Tokyo). 2015;68(5):334-338. doi:10.1038/ja.2014.153
Liu H, Yan C, Li C, You T, She Z. Naphthoquinone Derivatives with Anti-Inflammatory Activity from Mangrove-Derived Endophytic Fungus Talaromyces sp. SK-S009. Molecules. 2020;25(3):1-9. doi:10.3390/molecules25030576
Guoliang Z, Zhang X, Shah M, et al. Polyhydroxy p -Terphenyls from a Mangrove Endophytic. Mar Drugs. Published online 2021:1-10.
Jakočiūnas T, Klitgaard AK, Kontou EE, et al. Programmable Polyketide Biosynthesis Platform for Production of Aromatic Compounds in Yeast. Synth Syst Biotechnol. 2020;5(1):11-18. doi:10.1016/j.synbio.2020.01.004
Zhang W, Zhao B, Du L, Shen Y. Cytotoxic Polyketides with an Oxygen-Bridged Cyclooctadiene Core Skeleton from the Mangrove Endophytic Fungus Phomosis sp. A818. Molecules. 2017;22(9). doi:10.3390/molecules22091547
Wei C, Deng Q, Sun M, Xu J. Cytospyrone and Cytospomarin: Two New Polyketides Isolated from Mangrove Endophytic Fungus, Cytospora sp. Molecules. 2020;25(18):1-9. doi:10.3390/molecules25184224
Yu X, Müller WEG, Meier D, et al. Polyketide Derivatives from Mangrove Derived Endophytic Fungus Pseudopestalotiopsis theae. Mar Drugs. 2020;18(2):1-15. doi:10.3390/md18020129
Li T, Wang Y, Li L, et al. New Cytotoxic Cytochalasans from a Plant-Associated Fungus Chaetomium globosum Kz-19. Mar Drugs. 2021;19(8):1-10. doi:10.3390/md19080438
Wang CF, Ma J, Jing QQ, et al. Integrating Activity-Guided Strategy and Fingerprint Analysis to Target Potent Cytotoxic Brefeldin A from a Fungal Library of the Medicinal Mangrove Acanthus ilicifolius. Mar Drugs. 2022;20(7). doi:10.3390/md20070432
Feng T, Wei C, Deng X, Chen D, Wen Z, Xu J. Epigenetic Manipulation Induced Production of Immunosuppressive Chromones and Cytochalasins from the Mangrove Endophytic Fungus Phomopsis asparagi DHS-48. Mar Drugs. 2022;20(10). doi:10.3390/md20100616
Vermerris W, Nicholson R. Phenolic Compound Biochemistry. Choice Rev Online. 2007;45(02):45-0882-45-0882. doi:10.5860/choice.45-0882
Wang J, Lu Z, Liu P, et al. Cytotoxic Polyphenols from the Fungus Penicillium expansum 091006 Endogenous with the Mangrove Plant Excoecaria agallocha. Planta Med. 2012;78(17):1861-1866. doi:10.1055/s-0032-1315395
Wang J, Cox DG, Ding W, Huang G, Lin Y, Li C. Three New Resveratrol Derivatives from the Mangrove Endophytic Fungus Alternaria sp. Mar Drugs. 2014;12(5):2840-2850. doi:10.3390/md12052840
Liu J, Xu M, Zhu MY, Feng Y. Chemoreversal Metabolites from the Endophytic Fungus Penicillium citrinum Isolated from a Mangrove Avicennia marina. Nat Prod Commun. 2015;10(7):1203-1205. doi:10.1177/1934578x1501000717
Chen S, Chen D, Cai R, et al. Cytotoxic and Antibacterial Preussomerins from the Mangrove Endophytic Fungus Lasiodiplodia theobromae ZJ-HQ1. J Nat Prod. 2016;79(9):2397-2402. doi:10.1021/acs.jnatprod.6b00639
Li F, Guo W, Che Q, Zhu T, Gu Q, Li D. Versicones E-H and Arugosin K Produced by the Mangrove-Derived Fungus Aspergillus versicolor HDN11-84. J Antibiot (Tokyo). 2017;70(2):174-178. doi:10.1038/ja.2016.95
Yu G, Wu G, Sun Z, et al. Cytotoxic Tetrahydroxanthone Dimers from the Mangrove-Associated Fungus Aspergillus versicolor HDN1009. Mar Drugs. 2018;16(9). doi:10.3390/md16090335
Tiwari P, Bae H. Endophytic Fungi: Key Insights, Emerging Prospects, and Challenges in Natural Product Drug Discovery. Microorganisms. 2022;10(2). doi:10.3390/microorganisms10020360
Ebrahim W, Kjer J, El Amrani M, et al. Pullularins E and F, Two New Peptides from the Endophytic Fungus Bionectria ochroleuca Isolated from the Mangrove Plant Sonneratia caseolaris. Mar Drugs. 2012;10(5):1081-1091. doi:10.3390/md10051081
Deng CM, Liu SX, Huang CH, Pang JY, Lin YC. Secondary Metabolites of a Mangrove Endophytic Fungus Aspergillus terreus (No. GX7-3B) from the South China Sea. Mar Drugs. 2013;11(7):2616-2624. doi:10.3390/md11072616
Zhu M, Yang Z, Feng H, et al. Trichodermamides D-F, Heterocyclic Dipeptides with a Highly Functionalized 1,2-Oxazadecaline Core Isolated from the Endophytic Fungus: Penicillium janthinellum HDN13-309. RSC Adv. 2017;7(76):48019-48024. doi:10.1039/c7ra10389a
Niu S, He J, Huang S, et al. Phaeosphamides A and B, Cytotoxic Cyclodecadepsipeptides from the Mangrove-Derived Fungus Phaeosphaeriopsis sp. S296. Mar Drugs. 2022;20(10):1-11. doi:10.3390/md20100591
Zorrilla JG, Evidente A. Structures and Biological Activities of Alkaloids Produced by Mushrooms, a Fungal Subgroup. Biomolecules. 2022;12(8):1-25. doi:10.3390/biom12081025
Zhu F, Wu J, Chen G, Lu W, Pan J. Biosynthesis, Characterization and Biological Evaluation of Fe(III) and Cu(II) Complexes of Neoaspergillic Acid, a Hydroxamate Siderophore Produced by Co-Cultures of Two Marine-Derived Mangrove Epiphytic Fungi. Nat Prod Commun. 2011;6(8):1137-1140. doi:10.1177/1934578x1100600824
Zhou ZF, Kurtan T, Yang XH, et al. ChemInform Abstract: Penibruguieramine A, a Novel Pyrrolizidine Alkaloid from the Endophytic Fungus Penicillium sp. GD6 Associated with Chinese Mangrove Bruguiera gymnorrhiza. ChemInform. 2014;45(33). doi:10.1002/chin.201433224
Huang S, Chen H, Li W, Zhu X, Ding W, Li C. Bioactive Chaetoglobosins from the Mangrove Endophytic Fungus Penicillium chrysogenum. Mar Drugs. 2016;14(10):1-12. doi:10.3390/md14100172
Wu Y, Chen S, Liu H, et al. Cytotoxic Isocoumarin Derivatives from the Mangrove Endophytic Fungus Aspergillus sp. HN15-5D. Arch Pharm Res. 2019;42(4):326-331. doi:10.1007/s12272-018-1019-1
Chen Y, Wang G, Yuan Y, et al. Metabolites With Cytotoxic Activities From the Mangrove Endophytic Fungus Fusarium sp. 2ST2. Front Chem. 2022;10(February):1-8. doi:10.3389/fchem.2022.842405
Liu Y, Stuhldreier F, Kurtán T, et al. Daldinone Derivatives from the Mangrove-Derived Endophytic Fungus: Annulohypoxylon sp. RSC Adv. 2017;7(9):5381-5393. doi:10.1039/c6ra27306h
Teiten MH, Mack F, Debbab A, Aly AH, Dicato M, Proksch P, Diederich M. Anticancer Effect of Altersolanol A, a Metabolite Produced by the Endophytic Fungus Stemphylium globuliferum, Mediated by its Pro-apoptotic and Anti-invasive Potential via the Inhibition of NF-κB Activity. 2013;21(13):3850-8. doi: 10.1016/j.bmc.2013.04.024
Feng S, Wang W. Bioactivities and Structure-Activity Relationships of Natural Tetrahydroanthraquinone Compounds: A Review. 2020;11(799):1-10. doi: 10.3389/fphar.2020.00799
Balasundram N, Sundram K, Samman S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. 2006;99:191-203. doi:10.1016/j.foodchem.2005.07.042
Downloads
Submitted
Accepted
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Jurnal Ilmiah Medicamento

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.





















