Combination of Cocrystal and Ball Milling Techniques to Improve Etoricoxib Dissolution
DOI:
https://doi.org/10.36733/medicamento.v10i1.7561Keywords:
ball milling, cocrystal, etoricoxib, oxalic acid, Tween 80Abstract
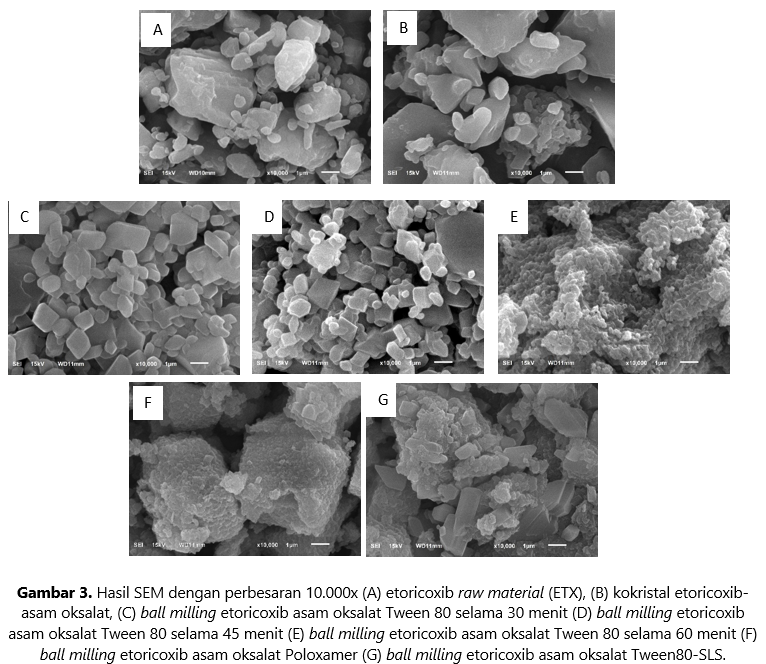
Etoricoxib (ETX) is a selective COX-2 anti-inflammatory drug classified in BCS class II. This study aims to enhance the dissolution rate of etoricoxib through a combination of co-crystal formation and ball milling conducted in-situ and ex-situ. Optimization was done by varying milling times and types of stabilizers, including Tween 80 (ETX-OXA-BM-T), Poloxamer 188 (ETX-OXA-BM-P), and a combination of Tween 80-sodium lauryl sulfate (SLS) (ETX-OXA-BM-T-S). In-situ experiments yielded a very low yield (<10%) and failed to produce co-crystals, thus deeming them unsuitable for continuation. Meanwhile, the ex-situ process showed more potential, leading to further evaluation using Differential Scanning Calorimetry (DSC), Powder X-Ray Diffractometry (PXRD), and Scanning Electron Microscope (SEM). DSC analysis showed endothermic peaks at 130°C for ETX and around 179 - 180°C for ETX-OXA and its derivatives. PXRD diffractograms for ETX-OXA and its derivatives exhibited similar peaks, differing from ETX. SEM analysis indicated that ETX-OXA-BM-T with 60 minutes of milling resulted in nanometer-sized particles, while the use of Poloxamer 188 and the combination of Tween 80-SLS produced particle sizes > 1 µm. ETX-OXA-BM-T showed the highest increase in solubility in all media. The dissolution results of ETX-OXA-BM-T showed improvement in phosphate buffer pH 6.8, while no significant differences were observed in pH 1.2 and 4.5 buffers. This study demonstrates that the combination of co-crystal formation and ex-situ ball milling is a potential approach to enhancing the dissolution rate of etoricoxib.
References
Arfan AR, Ilmiawati A, Sugita P. Optimization and synthesis of etoricoxib-loaded low molecular weight chitosan nanoparticles. Ciencia Rural. 2022;52(11). doi:10.1590/0103-8478cr20210656
Malviya R, Sharma PK, Dubey SK. Efficiency of self‐assembled etoricoxib containing polyelectrolyte complex stabilized cubic nanoparticles against human cancer cells. Precision Medical Sciences. 2020;9(1):9-22. doi:10.1002/prm2.12004
Dave V, Srivastava P, Tak K, Sharma S. PEG-PLGA- hybrid nanoparticles loaded with etoricoxib–phospholipid complex for effective treatment of inflammation in rat model. J Microencapsul. 2019;36(3):236-249. doi:10.1080/02652048.2019.1617362
Wang Y, Wang L, Zhang F, et al. Structure analysis and insight into hydrogen bond and van der waals interactions of etoricoxib cocrystals and cocrystal solvate. J Mol Struct. 2022;1258. doi:10.1016/j.molstruc.2022.132665
Sapkal SB, Adhao VS, Thenge RR, Darakhe RA, Shinde SA, Shrikhande VN. Formulation and characterization of solid dispersions of etoricoxib using natural polymers. Turk J Pharm Sci. 2020;17(1):7-19. doi:10.4274/tjps.galenos.2018.04880
Banerjee M, Nimkar K, Naik S, Patravale V. Unlocking the potential of drug-drug cocrystals – A comprehensive review. Journal of Controlled Release. 2022;348:456-469. doi:10.1016/j.jconrel.2022.06.003
Yan Y, Wang L, Si Z, Zhang X, Yuan W. A novel cocrystal of metformin hydrochloride with citric acid: Systematic synthesis and computational simulation. European Journal of Pharmaceutics and Biopharmaceutics. 2022;179:37-46. doi:10.1016/j.ejpb.2022.08.013
Guo M, Sun X, Chen J, Cai T. Pharmaceutical cocrystals: A review of preparations, physicochemical properties and applications. Acta Pharm Sin B. 2021;11(8):2537-2564. doi:10.1016/j.apsb.2021.03.030
Panzade P, Shendarkar G, Kulkarni D, Shelke S. Solid state characterization and dissolution enhancement of nevirapine cocrystals. Adv Pharm Bull. 2021;11(4):772-776. doi:10.34172/APB.2021.087
Liu M, Hong C, Li G, Ma P, Xie Y. The generation of myricetin-nicotinamide nanococrystals by top down and bottom up technologies. Nanotechnology. 2016;27(39). doi:10.1088/0957-4484/27/39/395601
Shen D, Jin T, Xiao Y, Zhu X, Hua Y. Preparation of pazopanib-fumarate disodium glycyrrhizinate nanocrystalline micelles by liquid-assisted ball milling. European Journal of Pharmaceutical Sciences. 2023;188. doi:10.1016/j.ejps.2023.106530
Martínez LM, Cruz-Angeles J, Vázquez-Dávila M, et al. Mechanical Activation by Ball Milling as a Strategy to Prepare Highly Soluble Pharmaceutical Formulations in the Form of Co-Amorphous, Co-Crystals, or Polymorphs. Pharmaceutics. 2022;14(10). doi:10.3390/pharmaceutics14102003
Wang Y, Wang L, Zhang F, et al. Structure analysis and insight into hydrogen bond and van der waals interactions of etoricoxib cocrystals and cocrystal solvate. J Mol Struct. 2022;1258:132665. doi:10.1016/j.molstruc.2022.132665
Nugrahani I, Auli WN. Diclofenac-proline nano-co-crystal development, characterization, in vitro dissolution and diffusion study. Heliyon. 2020;6(9). doi:10.1016/j.heliyon.2020.e04864
Missouri State University and Ozarks Environmental and Water Resources Institute (OEWRI). Standard Operating Procedure for: LS 13 320 Laser Diffraction Particle Size Analyzer Operation.; 2008.
Unique IGNP, Nurono S, Nugraha YP. Modifikasi Sifat Fisikokimia Telmisartan melalui Pembentukan Garam. Bandung Institute of Technology; 2023.
Singh S, Mishra A, Verma A, Ghosh AK, Mishra AK. A simple ultraviolet spectrophotometric method for the determination of etoricoxib in dosage formulations. J Adv Pharm Technol Res. 2012;3(4):237-240. doi:10.4103/2231-4040.104715
Huang Z, Staufenbiel S, Bodmeier R. Combination of co-crystal and nanocrystal techniques to improve the solubility and dissolution rate of poorly soluble drugs. Pharm Res. 2022;39(5):949-961. doi:10.1007/s11095-022-03243-9
Shan Chow P, Lau G, Kiong Ng W, Vangala VR. Stability of Pharmaceutical Cocrystal During Milling: A Case Study of 1:1 Caffeine-Glutaric Acid 2. Crystal Growth& Design. 2017;17(8):4064-4071.
Santos JAV, Baptista JA, Santos IC, et al. Pharmaceutical nanococrystal synthesis: A novel grinding approach. CrystEngComm. 2022;24(5):947-961. doi:10.1039/d1ce00407g
Vollath D. Agglomerates of nanoparticles. Beilstein Journal of Nanotechnology. 2020;11:854-857. doi:10.3762/BJNANO.11.70
Wahyuni R, Makmur I, Putri SA. Optimization of Ball Ratio in Planetary Ballmill in Nimodipine-poloxamer 188 Nanoparticle Formulation Process. International Journal of Pharmaceutical Sciences and Medicine. 2022;7(9):1-9. doi:10.47760/ijpsm.2022.v07i09.001
Putri T, Saputra IS, Saputro AH, Permana YN, Yulizar Y. Sodium Laureth Sulfate (SLS) Decorated α-PBO Nanocrystals : Optical, Structure, and Morphology Properties. Jurnal Sains Materi Indonesia. 2021;22(2):71-76.
Weldon DG, Hemminger WF, Flammersheim HJ. Differential Scanning Calorimetry. Vol 31. Springer-Verlag Berlin Heidelberg; 2014. doi:10.1007/978-3-662-06710-9
National Center for Biotechnology Information. Oxalic acid dihydrate. PubChem Compound Summary for CID 61373, Oxalic acid dihydrate.
Grobelny P, Mukherjee A, Desiraju GR. Polymorphs and hydrates of Etoricoxib, a selective COX-2 inhibitor. CrystEngComm. 2012;14(18):5785-5794. doi:10.1039/c2ce06604a
Kurniawan C, Waluyo TB, Sebayang P, Pusat ), Fisika P. Analisis Ukuran Partikel Menggunakan Free Software Image-J.; 2011. http://rsb.info.nih.gov/ij/.
Liu M, Hong C, Li G, Ma P, Xie Y. The generation of myricetin-nicotinamide nanococrystals by top down and bottom up technologies. Nanotechnology. 2016;27(39). doi:10.1088/0957-4484/27/39/395601
Peltonen L, Hirvonen J. Pharmaceutical nanocrystals by nanomilling: Critical process parameters, particle fracturing and stabilization methods. Journal of Pharmacy and Pharmacology. 2010;62(11):1569-1579. doi:10.1111/j.2042-7158.2010.01022.x
Das A, Nayak AK, Mohanty B, Panda S. Solubility and Dissolution Enhancement of Etoricoxib by Solid Dispersion Technique Using Sugar Carriers. ISRN Pharm. 2011;2011:1-8. doi:10.5402/2011/819765
Md S, Alhakamy NA, Alharbi WS, et al. Development and evaluation of repurposed etoricoxib loaded nanoemulsion for improving anticancer activities against lung cancer cells. Int J Mol Sci. 2021;22(24). doi:10.3390/ijms222413284
Sugano K, Omori M, Watanabe T, Uekusa T, Oki J, Inoue D. Effects of coformer and polymer on particle surface solution- mediated phase transformation of cocrystals in aqueous media. Mol Pharm. 2020;17(10):3825-3836. doi:10.1021/acs.molpharmaceut.0c00587
Okumu A, DiMaso M, Löbenberg R. Computer simulations using GastroPlusTM to justify a biowaiver for etoricoxib solid oral drug products. European Journal of Pharmaceutics and Biopharmaceutics. 2009;72(1):91-98. doi:10.1016/j.ejpb.2008.10.019
Mitra A, Kesisoglou F, Dogterom P. Application of Absorption Modeling to Predict Bioequivalence Outcome of Two Batches of Etoricoxib Tablets. AAPS PharmSciTech. 2014;16(1):76-84. doi:10.1208/s12249-014-0194-8
Loh ZH, Samanta AK, Sia Heng PW. Overview of milling techniques for improving the solubility of poorly water-soluble drugs. Asian J Pharm Sci. 2014;10(4):255-274. doi:10.1016/j.ajps.2014.12.006
Downloads
Submitted
Accepted
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Sharon Susanto, Saleh Wikarsa, Yuda Prasetya Nugraha

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.





















