Proliferation of Nano Chitosan and Platelet Rich Plasma from Pre-osteoblast Cell with Ki67 as a Surrogate Marker in Vitro
DOI:
https://doi.org/10.36733/medicamento.v11i2.11435Keywords:
hydroxyapatite, nano-chitosan, pre-osteoblast, proliferation, PRPAbstract
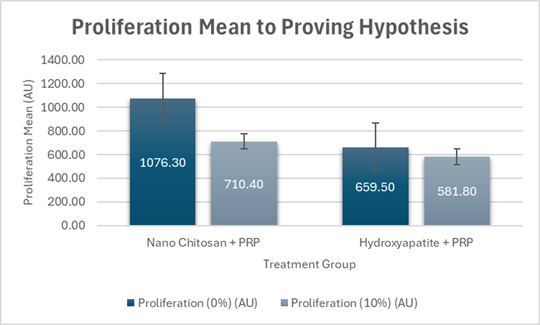
This study investigated the effect of nano-chitosan mix with platelet rich plasma (PRP) to proliferation rate of pre-osteoblast cell with incubation time used in vitro culture system. The culture media of pre-osteoblast cell MC3T3-E1 used alpha-MEM, 2mm L-glutamine, 1mm sodium pyruvate, 10% FBS and 10% pen strep in 25cm2 flask bottle and incubated in an incubator with 5% CO2 at a temperature of 37oC until the cell was confluent 70-80% and planting in well-24 to give treatments. Treatment was divided into two groups, nano-chitosan+PRP and hydroxyapatite+PRP. The proliferation of pre-osteoblast cells saw with immunocytochemical staining and proliferation of cells were counted and investigated with confocal laser scanning microscope (CLSM). The normality of sample data was analyzed with Shapiro-Wilk. Comparison test used independent sample t-test and one-way ANOVA (F-test). All data were analyzed with SPSS software. The experiment results showed that nano-chitosan+PRP can accelerate proliferation than hydroxyapatite+PRP of 0% and 10% concentrations. The independent sample t-test showed there were a significant difference (p=0.010<) from proliferation rate mean (0%) between treatment group nano-chitosan+PRP (1076.3±176.4au) and treatment group hydroxyapatite+PRP (659.5±272.7au) on five days incubation time, and proliferation mean (10%) between treatment group of nano-chitosan+PRP (710.3±109.7au) and hydroxyapatite+PRP (581.8±76.4au) on seven days incubation time. Based on proliferation mean (0%) and (10%), the treatment group of nano-chitosan+PRP with five- and seven-days incubation have higher mean than 0% and 10% on treatment group nano-chitosan and PRP and can accelerate bone healing with incubation time of five and seven days compared to treatment group of hydroxyapatite+PRP.
References
1. Lewusz-Butkiewicz K, Kaczor-Wiankowska K, Szmidt-Kadys M, Rogocka M, Lagocka R. Failed Non-Surgical Endodontic Treatment of First and Second Left Incisors and the Next Successful Apical Resection – A Case Report with Three-Year Follow-Up. J Radiol Case Rep. 2024;18(3):37-46. doi:10.3941/jrcr.v18i3.5259
2. Choi IA, Umemoto A, Mizuno M, Park-Min KH. Bone metabolism – an underappreciated player. npj Metab Heal Dis. 2024;2(1):12. doi:10.1038/s44324-024-00010-9
3. Raggatt LJ, Partridge NC. Cellular and Molecular Mechanisms of Bone Remodeling. J Biol Chem. 2010;285(33):25103-25108. doi:10.1074/jbc.R109.041087
4. Siddiqui JA, Partridge NC. Physiological Bone Remodeling: Systemic Regulation and Growth Factor Involvement. Physiology. 2016;31(3):233-245. doi:10.1152/physiol.00061.2014
5. Koirala P, Bhattarai P, Sriprablom J, Zhang R, Nirmal S, Nirmal N. Recent progress of functional nano-chitosan in pharmaceutical and biomedical applications: An updated review. Int J Biol Macromol. 2025;285:138324. doi:10.1016/j.ijbiomac.2024.138324
6. Chandra A, Lan S, Zhu J, Siclari VA, Qin L. Epidermal Growth Factor Receptor (EGFR) Signaling Promotes Proliferation and Survival in Osteoprogenitors by Increasing Early Growth Response 2 (EGR2) Expression. J Biol Chem. 2013;288(28):20488-20498. doi:10.1074/jbc.M112.447250
7. Baghersad S, Bolandi B, Imani R, Afaghi S, Davoudinia S. An Overview of PRP-Delivering Scaffolds for Bone and Cartilage Tissue Engineering. J Bionic Eng. 2024;21(2):674-693. doi:10.1007/s42235-023-00471-6
8. Akbar AF, Cahyaningrum SE. Characterization and Anti-Bacterial Activity Testing of the Nano Hydroxyapatite-Clove (Eugenia Caryophyllus) Against Streptococcus Mutans Bacteria. Indones J Chem Sci. 2022;11(1):1-8. doi:10.15294/ijcs.v11i1.51037
9. Zhu H, Song W, Deng Y. Hydroxyapatite extracted by animal bone image analysis in ionic liquid choline chloride-glycerol. EURASIP J Image Video Process. 2018;2018(1):56. doi:10.1186/s13640-018-0295-5
10. Gautam CR, Kumar S, Mishra VK, Biradar S. Synthesis, structural and 3-D architecture of lanthanum oxide added hydroxyapatite composites for bone implant applications: Enhanced microstructural and mechanical properties. Ceram Int. 2017;43(16):14114-14121. doi:10.1016/j.ceramint.2017.07.150
11. Steller D, Herbst N, Pries R, Juhl D, Hakim SG. Positive impact of Platelet-rich plasma and Platelet-rich fibrin on viability, migration and proliferation of osteoblasts and fibroblasts treated with zoledronic acid. Sci Rep. 2019;9(1):8310. doi:10.1038/s41598-019-43798-z
12. Wang X, Zhang Y, Choukroun J, Ghanaati S, Miron RJ. Effects of an injectable platelet-rich fibrin on osteoblast behavior and bone tissue formation in comparison to platelet-rich plasma. Platelets. 2018;29(1):48-55. doi:10.1080/09537104.2017.1293807
13. Bartonickova E, Vojtisek J, Tkacz J, et al. Porous HA/Alumina composites intended for bone-tissue engineering. Mater Tehnol. 2017;51(4):631-636. doi:10.17222/mit.2016.191
14. Souza JCM, Sordi MB, Kanazawa M, et al. Nano-scale modification of titanium implant surfaces to enhance osseointegration. Acta Biomater. 2019;94:112-131. doi:10.1016/j.actbio.2019.05.045
15. Mangkuasih SM, Rohmawati L. Sintesis Hidroksiapatit dari Tulang Ikan Sapu-Sapu (Hypostomus plecostomus) dengan Metode Presipitasi. J Teor dan Apl Fis. 2021;9(2):229. doi:10.23960/jtaf.v9i2.2818
16. Carlos Rodríguez-Merchán E. The Molecular Mechanisms of Bone Healing. Int J Mol Sci. 2021;22:767. https://doi.org/10.3390/ijms22020767
17. Mizoguchi T, Ono N. The diverse origin of bone-forming osteoblasts. J Bone Miner Res. 2020;36(8):1432-1447. doi:10.1002/jbmr.4410
18. Mehreen A, Faisal M, Zulfiqar B, et al. Connecting Bone Remodeling and Regeneration: Unraveling Hormones and Signaling Pathways. Biology (Basel). 2025;14(3):274. doi:10.3390/biology14030274
19. Hersanti H, Choiriah WS, Rizkie L, Putri SNS. Effects of Chitosan and Silica Nanoparticles Against the Development and Growth of Red Chilli Anthracnose Disease Colletotrichum sp. Pakistan J Biol Sci. 2022;25(8):748-754. doi:10.3923/pjbs.2022.748.754
20. Mascarenhas R, Hegde S, Manaktala N. Chitosan nanoparticle applications in dentistry: a sustainable biopolymer. Front Chem. 2024;12(April):1-21. doi:10.3389/fchem.2024.1362482
21. Ikono R, Mardliyati E, Agustin IT, et al. Chitosan—PRP nanosphere as a growth factors slow releasing device with superior antibacterial capability. Biomed Phys Eng Express. 2018;4(4):045026. doi:10.1088/2057-1976/aac9f8
22. Dai L, Wang X, Zhang J, Li C. Application of Chitosan and Its Derivatives in Postharvest Coating Preservation of Fruits. Foods. 2025;14(8):1318. doi:10.3390/foods14081318
23. Adiletta G, Di Matteo M, Petriccione M. Multifunctional Role of Chitosan Edible Coatings on Antioxidant Systems in Fruit Crops: A Review. Int J Mol Sci. 2021;22(5):2633. doi:10.3390/ijms22052633
24. Albanese A, Licata ME, Polizzi B, Campisi G. Platelet-rich plasma (PRP) in dental and oral surgery: From the wound healing to bone regeneration. Immun Ageing. 2013;10(1):1. doi:10.1186/1742-4933-10-23
25. Giannelli A, Forte M, D’Albis G, et al. Utilization of Platelet-Rich Plasma in Oral Surgery: A Systematic Review of the Literature. J Clin Med. 2025;14(8):2844. doi:10.3390/jcm14082844
26. Aguilar A, Zein N, Harmouch E, et al. Application of Chitosan in Bone and Dental Engineering. Molecules. 2019;24(16):3009. doi:10.3390/molecules24163009
27. George Ittycheria P, George T, John M, et al. Application of Hydroxyapatite in Regenerative Dentistry. In: Novel Biomaterials for Tissue Engineering. IntechOpen; 2024. doi:10.5772/intechopen.112387
28. Supandi SK, Susilahati NLDA, Lubna L, Rezkika YF, Krismariono A, Maduratna E. Micro Hydroxyapatite in Bone Regeneration: A Literature Review. Res J Pharm Technol. 2024;17(2):591-594. doi:10.52711/0974-360X.2024.00092
Downloads
Submitted
Accepted
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Dewa Made Wedagama

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.





















