Efektivitas dan Efek Samping pada Terapi Multi-Drug-Resistant Tuberculosis (MDR-TB): Kajian Literatur
DOI:
https://doi.org/10.36733/medicamento.v11i1.9685Kata Kunci:
amikasin, bedaquiline, fluorokuinolon, MDR-TBAbstrak
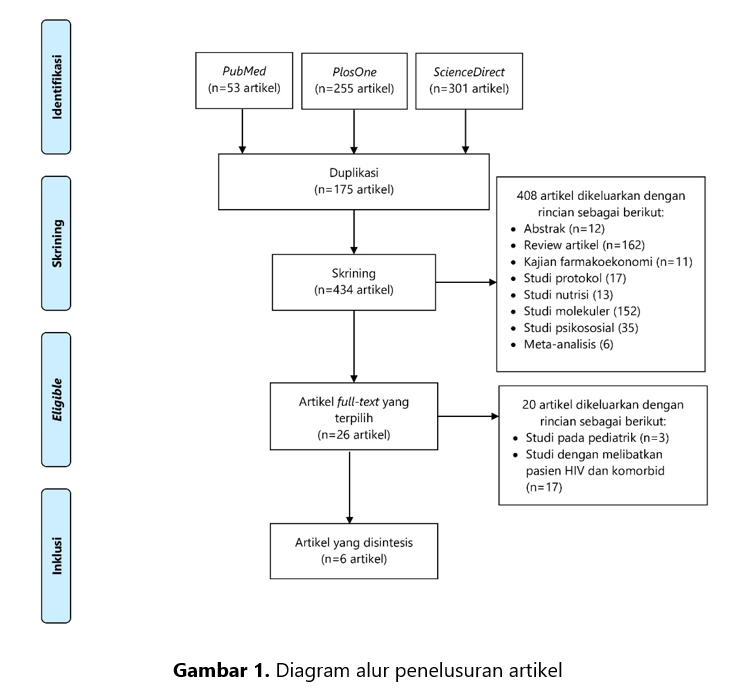
Multi-drug resistant tuberkulosis (MDR-TB) menyebabkan angka kesembuhan menjadi menurun. Secara global, angka kejadian MDR-TB pada tahun 2015-2020 relatif stabil namun terjadi peningkatan pada tahun 2021. Pada tahun 2020, World Health Organization (WHO) memperkirakan terdapat 437.000 kasus MDR-TB di dunia dan jumlah tersebut mengalami peningkatan menjadi 450.000 kasus pada tahun 2021. Adanya berbagai macam rejimen terapi yang direkomendasikan WHO maka perlu dilakukan sebuah kajian literatur yang memberikan gambaran tentang efektivitas terapi dan efek samping penggunaan obat pada kasus MDR-TB. Kajian ini akan memberikan keterbaruan informasi dan dapat dijadikan referensi untuk mengidentifikasi dan mengelola efek samping secara dini. Kajian literatur ini bersifat narrative review (kajian naratif) dengan mengumpulkan dan menelaah informasi dari berbagai artikel internasional tentang efektivitas dan efek samping pada penatalaksanaan MDR-TB. Pencarian artikel menggunakan database PubMed, PlosOne, dan ScienceDirect sejak Januari 2014 hingga Juni 2024. Sebanyak enam artikel relevan dari total 609 artikel yang disintesis. Secara deskriptif, efektivitas terapi MDR-TB menggunakan berbagai rejimen terapi dengan obat-obat yang direkomendasikan WHO menunjukkan angka kesembuhan yang tinggi (cured>50%). Angka kejadian efek samping pada terapi MDR-TB lebih kecil dibandingkan dengan efektivitas terapi. Namun, pada penelitian yang dilakukan di Rumah sakit di Wuhan Jinyintan-China pada periode Juli 2019-Desember 2020, menunjukkan adanya efek samping yang timbul pada semua subjek penelitian. Efek samping tersebut antara lain: mual dan muntah akibat penggunaan protionamide, gatifloksasin, dan etambutol; hiperurisemia akibat penggunaan pirazinamid, dan hiperpigmentasi akibat klofazimin. Pemilihan rejimen terapi disarankan berdasarkan pada hasil pemeriksaan kultur, kondisi pasien, serta ketersediaan obat di masing-masing negara.
Referensi
World Health Organization. Global tuberculosis report 2019 (World Health Organization, 2019). Published online 2019.
World Health Organization. Global Tuberculosis Report 2022.; 2022.
Kemenkes. Laporan Program Penanggulangan Tuberkulosis Tahun 2021. Kementerian Kesehatan RI; 2021.
World Health Organization. WHO Consolidated Guidelines on Tuberculosis.; 2022.
Agyeman AA, Ofori-Asenso R. Tuberculosis—an overview. Public Heal Emerg. 2016;1(November):37-37. doi:10.21037/phe.2016.10.04
Bansal R, Sharma D, Singh R. Tuberculosis and its Treatment: An Overview. Mini-Reviews Med Chem. 2016;18(1):58-71. doi:10.2174/1389557516666160823160010
World Health Organization. Global tuberculosis report 2015. Published online 2015.
The Working Group for New TB Drugs. No Title. Clinical Pipeline. 2016. Accessed July 31, 2024. https://www.newtbdrugs.org/pipeline/clinical?field_advancing_value=1&field_submitted_for_registration_value%5B%5D=1
The U.S. Food and Drug Administration. No Title. FDA approves new drug for treatment-resistant forms of tuberculosis that affects the lungs. 2019. Accessed July 30, 2024. https://www.fda.gov/news-events/press-announcements/fda-approves-new-drug-treatment-resistant-forms-tuberculosis-affects-lungs
The U.S. Food and Drug Administration. No Title. Drug Approval Package. 2020. Accessed July 31, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2000/21024S5_Priftin.cfm
Rahman N, Meng SK, Ahmed IA. Current Review of the Use of Linezolid in the Treatment of Multidrug-Resistant Tuberculosis: Effectiveness and Management of Side Effects. Int J Biotechnol Biomed. 2024;1(1):89-102. doi:https://doi.org/10.31674/ijbb.2024.v01i01.005
Ari Kusuma Yana IGA, Herawati F. Efektivitas Dan Keamanan Terapi dengan Rejimen Bedaquiline dalam Terapi Multidrug-Resistant Tuberculosis (TB-MDR): Kajian Sistematis. Pharm J Indones. 2022;7(2):129-138. doi:10.21776/ub.pji.2022.007.02.8
Thariqulhaq MF, Wahyono TYM. The Effectiveness And Safety Of Bedaquiline-Containing Regimens In The Treatment Of Patients With Multi-Drug Resistant Tuberculosis (Mdr-Tb): A Systematic Literature Review. J EduHealth. 2023;14(03):1382-1392. doi:https://doi.org/10.54209/jurnaleduhealth.v14i3.2678
Sari E, Zakiah N, Santoso P, Barliana MI. EFektivitas Terapi Bedaquilin Dan Delamanid Pada Pengobatan Multidrug-Resistant Tuberculosis (MDR-TB): Sebuah Review. J Sains dan Teknol Farm Indones. 2020;9(1).
Mahardani PN, Wati DK, Siloam A, Savitri NPA, Manggala AK. Effectiveness and safety of short-term regimen for multidrug-resistant tuberculosis treatment: a systematic review of cohort studies. Oman Med J. 2022;37(1):e337. doi:https://doi.org/10.5001/omj.2021.64
Wu S, Zhang Y, Sun F, et al. Adverse events associated with the treatment of multidrug-resistant tuberculosis: A systematic review and meta-analysis. Am J Ther. 2016;23(2):e521-e530. doi:10.1097/01.mjt.0000433951.09030.5a
Zeng X, Zhang Y, Kwong JSW, et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta‐analysis, and clinical practice guideline: a systematic review. J Evid Based Med. 2015;8(1):2-10. doi:https://doi.org/10.1111/jebm.12141
Sun W, Wu Z, Zhou Y, et al. A highly effective and inexpensive standardized treatment of multidrug-resistant tuberculosis: a multicenter prospective study in China. BMC Infect Dis. 2021;21:1-9. doi:https://doi.org/10.1186/s12879-021-06553-2
Yao G, Zhu M, Nie Q, et al. Improved outcomes following addition of bedaquiline and clofazimine to a treatment regimen for multidrug-resistant tuberculosis. J Int Med Res. 2023;51(1):03000605221148416. doi:10.1177/03000605221148416
D’Ambrosio L, Bothamley G, Luna JAC, et al. Team approach to manage difficult-to-treat TB cases: experiences in Europe and beyond. Pulmonology. 2018;24(2):132-141. doi:https://doi.org/10.1016/j.rppnen.2017.10.005
Tiberi S, Buchanan R, Caminero JA, et al. The challenge of the new tuberculosis drugs. Presse Med. 2017;46(2):e41-e51. doi:http://dx.doi.org/10.1016/j.lpm.2017.01.016
Sun F, Li Y, Chen Y, et al. Introducing molecular testing of pyrazinamide susceptibility improves multidrug-resistant tuberculosis treatment outcomes: a prospective cohort study. Eur Respir J. 2019;53(3). doi:10.1183/13993003.01770-2018
Sangsayunh P, Sanchat T, Chuchottaworn C, Cheewakul K, Rattanawai S. The use of BPaL containing regimen in the MDR/PreXDR TB treatments in Thailand. J Clin Tuberc Other Mycobact Dis. 2024;34:100408. doi:10.1016/j.jctube.2023.100408
Nie Q, Tao L, Li Y, et al. High-dose gatifloxacin-based shorter treatment regimens for MDR/RR-TB. Int J Infect Dis. 2022;115:142-148. doi:10.1016/j.ijid.2021.11.037
Munir MK, Saeed MS, Haider SZ, Shamim S. Comparison of short term and long term multidrug resistant tuberculosis treatment outcomes in tertiary care settings. J King Saud Univ. 2024;36(4):103133. doi:10.1183/13993003.01770-2018
Bhanu MLS. Anti-Tuberculosis Drugs and Mechanisms of Action: Review. IJ Infect Disea. 2023;4(2):1-7.
Kwon YS, Jeong BH, Koh WJ. Tuberculosis: clinical trials and new drug regimens. Curr Opin Pulm Med. 2014;20(3):280-286. doi:10.1097/MCP.0000000000000045
Hooper DC, Jacoby GA. Topoisomerase inhibitors: fluoroquinolone mechanisms of action and resistance. Cold Spring Harb Perspect Med. 2016;6(9):a025320. doi:10.1101/cshperspect.a025320
Chauhan A, Kumar M, Kumar A, Kanchan K. Comprehensive review on mechanism of action, resistance and evolution of antimycobacterial drugs. Life Sci. 2021;274:119301. doi:10.1016/j.lfs.2021.119301
Jones NT, Abadie R, Keller CL, et al. Treatment and toxicity considerations in tuberculosis: A narrative review. Cureus. 2024;16(6). doi:10.7759/cureus.62698
Nugraha RV, Yunivita V, Santoso P, Aarnoutse RE, Ruslami R. Clofazimine as a treatment for multidrug-resistant tuberculosis: a review. Sci Pharm. 2021;89(2):19. doi:https://doi.org/10.3390/scipharm89020019
Hong H, Dowdy DW, Dooley KE, et al. Risk of hearing loss among multidrug-resistant tuberculosis patients according to cumulative aminoglycoside dose. Int J Tuberc Lung Dis. 2020;24(1):65-72. doi:https://doi.org/10.5588/ijtld.19.0062
Unduhan
Telah diserahkan
Diterima
Diterbitkan
Cara Mengutip
Terbitan
Bagian
Lisensi
Hak Cipta (c) 2025 Dwi Arymbhi Sanjaya, Herleeyana Meriyani, Rr. Asih Juanita, Nyoman Budiartha Siada, Lusy Noviani

Artikel ini berlisensi Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

















