Antibacterial Activity Test Of Sangu Bulung Ethanol Extract (Gracilaria Sp.) Against Gram Negative Bacteria Pseudomonas aeruginosa
DOI:
https://doi.org/10.36733/usadha.v2i3.7262Keywords:
gracilaria sp, pseudomonas aeruginosa, ultrasonic extract, zone of inhibitionAbstract
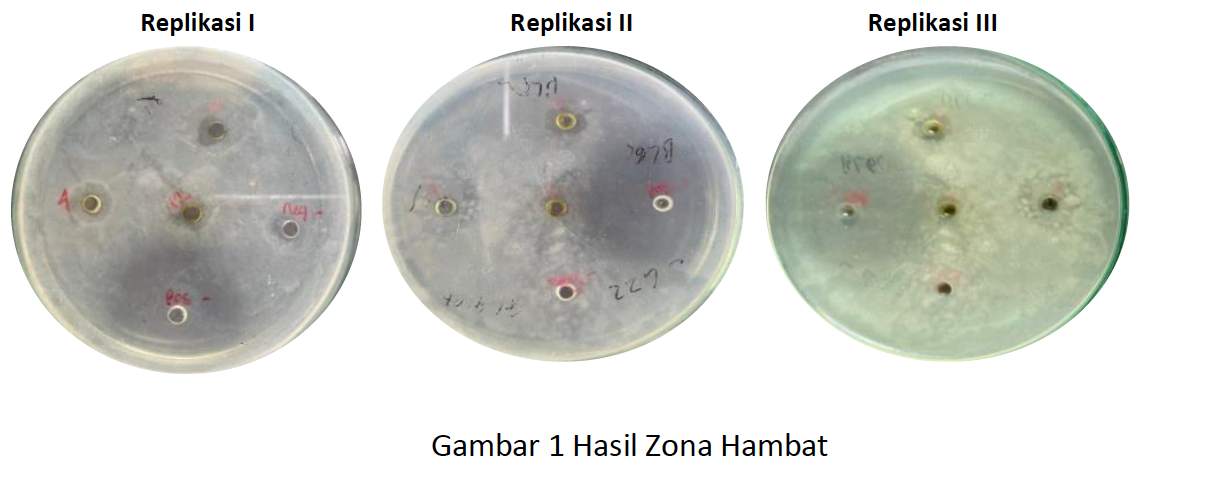
Pseudomonas aeruginosa is a gram-negative bacterium that is pathogenic to humans, especially in causing nosocomial infections. This bacterium has resistance to several classes of antibiotics, one of which is carbapenem and cephalosporin class antibiotics. The development of new antibiotics is an alternative in solving this case. Seaweed is one of the marine biota that has various benefits to be developed due to its primary and secondary metabolite compounds. Secondary metabolites in seaweed are used as a source of bioactive metabolites which have broad antiviral, antifungal and antibacterial activities. Bulung sangu (Gracilaria sp.) is one of the seaweeds that has biological activity as an antibacterial. This study aims to determine the antibacterial effectiveness of 70% ethanol extract of bulung sangu (Gracilaria sp.) on the growth of Pseudomonas aeruginosa bacteria. Sangu bamboo shoots were obtained from the southern Denpasar area (Bali). Bulung sangu viscous extract was extracted using 70% ethanol by ultrasonic method for 5 minutes at 40oC. Phytochemical screening of the extract was carried out to determine the content of secondary metabolite compounds it had. Antibacterial activity testing was carried out using the well diffusion method using 3 extract concentrations namely 4%, 8% and 12%. The positive and negative controls used were chloramphenicol antibiotics and sterile aquadest. Inhibition zones were observed and expressed as mean ± standard deviation (mm). The results were statistically analyzed using Kruskal-Wallis with a 95% confidence level. The inhibition zones generated from the positive control, concentrations of 4%, 8% and 12% could inhibit Pseudomonas aeruginosa bacteria with the following average diameters of the inhibition zones of 25.67mm, 2.00mm, 2.67mm and 2.67mm in the weak inhibition zone category.
References
[1].
Siregar AF, Sabdono A, Pringgenies D. Potensi antibakteri ekstrak rumput laut terhadap bakteri penyakit kulit Pseudomonas aeruginosa, Staphylococcus epidermidis, dan Micrococcus luteus. J Mar Res. 2012;1(2):83–5. doi:10.14710/jmr.v1i2.2032.
[2].
Sinurat AAP, Renta PP, Herliany NE, Negara BF, Purnama D. Uji aktivitas antibakteri ekstrak metanol rumput laut Gracilaria edulis terhadap bakteri Aeromonas hydrophila. J Enggano. 2019;4(1):105–14. doi:10.31186/jenggano.4.1.105-114.
[3].
Gita Bhernama B. Aktivitas antibakteri sabun padat yang mengandung ekstrak etanol rumput laut Gracilaria sp. terhadap bakteri Staphylococcus aureus. Pena Akuatika. 2020;19(1):34–44. doi:10.31941/penaakuatika.v19i1.1060.
[4].
Wulansari A, Aqlinia M, Wijanarka, Raharjo B. Isolasi bakteri endofit dari tanaman bangle (Zingiber cassumunar Roxb.) dan uji aktivitas antibakterinya terhadap bakteri penyebab penyakit kulit Staphylococcus epidermidis dan Pseudomonas aeruginosa. Berk Bioteknol. 2019;2(2).
[5].
Padmasari P, Astuti K, Warditiani N. Skrining fitokimia ekstrak etanol 70% rimpang bangle (Zingiber purpureum Roxb.). Chromatographia. 1998;47(3–4):234. doi:10.1007/bf02466588.
[6].
Murniasih T. Metabolit sekunder dari spons sebagai bahan obat-obatan. J Pesisir dan Laut Trop. 2003;6(1):44. doi:10.35800/jplt.6.1.2018.20192.
[7].
Putri DM, Lubis SS. Skrining fitokimia ekstrak etil asetat daun kalayu (Erioglossum rubiginosum (Roxb.) Blum). Amina. 2020;2(3):120–5.
[8].
Li Y, Kong D, Fu Y, Sussman MR, Wu H. The effect of developmental and environmental
Uji Aktivitas Antibakteri Ekstrak Etanol Bulung Sangu (Gracilaria sp.) Terhadap Bakteri Gram
Negatif Pseudomonas aeruginosa
USADHA: Jurnal Integrasi Obat Tradisional • Vol.2 No.3. • 2023 • ISSN-e: 2963-2161
111
factors on secondary metabolites in medicinal plants. Plant Physiol Biochem. 2020;148:80–9. doi:10.1016/j.plaphy.2020.01.006.
[9].
Ningsih AW, Nurrosyidah IH, Hisbiyah A. Pengaruh perbedaan metode ekstraksi rimpang kunyit (Curcuma domestica) terhadap rendemen dan skrining fitokimia. J Pharm Anwar Med. 2018;2(2):49–57. doi:10.36932/jpcam.v2i2.27.
[10].
Putri AL, Kusdiyantini E. Isolasi dan identifikasi bakteri asam laktat dari pangan fermentasi berbasis ikan (Inasua) yang diperjualbelikan di Maluku, Indonesia. J Biol Trop. 2018;1(2):6–12. doi:10.14710/jbt.1.2.6-12.
[11].
Nurhayati LS, Yahdiyani N, Hidayatulloh A. Perbandingan pengujian aktivitas antibakteri starter yoghurt dengan metode difusi sumuran dan metode difusi cakram. J Teknol Has Peternak. 2020;1(2):41–6. doi:10.24198/jthp.v1i2.27537.
[12].
Putriawati NI, Agrijati D. Inventarisasi Bacillus thuringiensis dengan metode cawan sebar pada habitat hidup larva Anopheles sp. pada tanaman eceng gondok (Eichornia crassipes). J Anal Med Bio Sains. 2018;5(1):91–5.
[13].
Wahyuni, Karim SF. Uji aktivitas antibakteri ekstrak etanol daun kacapiring (Gardenia jasminoides Ellis) terhadap bakteri Streptococcus mutans. J Sains dan Kesehat. 2020;2(4):399–404.
Downloads
Published
Issue
Section
License
Copyright (c) 2023 Leoni Putri, Maria Malida Vernandes Sasadara, Erna Cahyaningsih, Puguh Santoso

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.




