Pengaruh Polimorfisme Gen terhadap Risiko Penyakit Gagal Ginjal Kronis: Narrative Review
DOI:
https://doi.org/10.36733/usadha.v3i3.10956Kata Kunci:
gagal ginjal kronis, genetik, polimorfose gen, SNPAbstrak
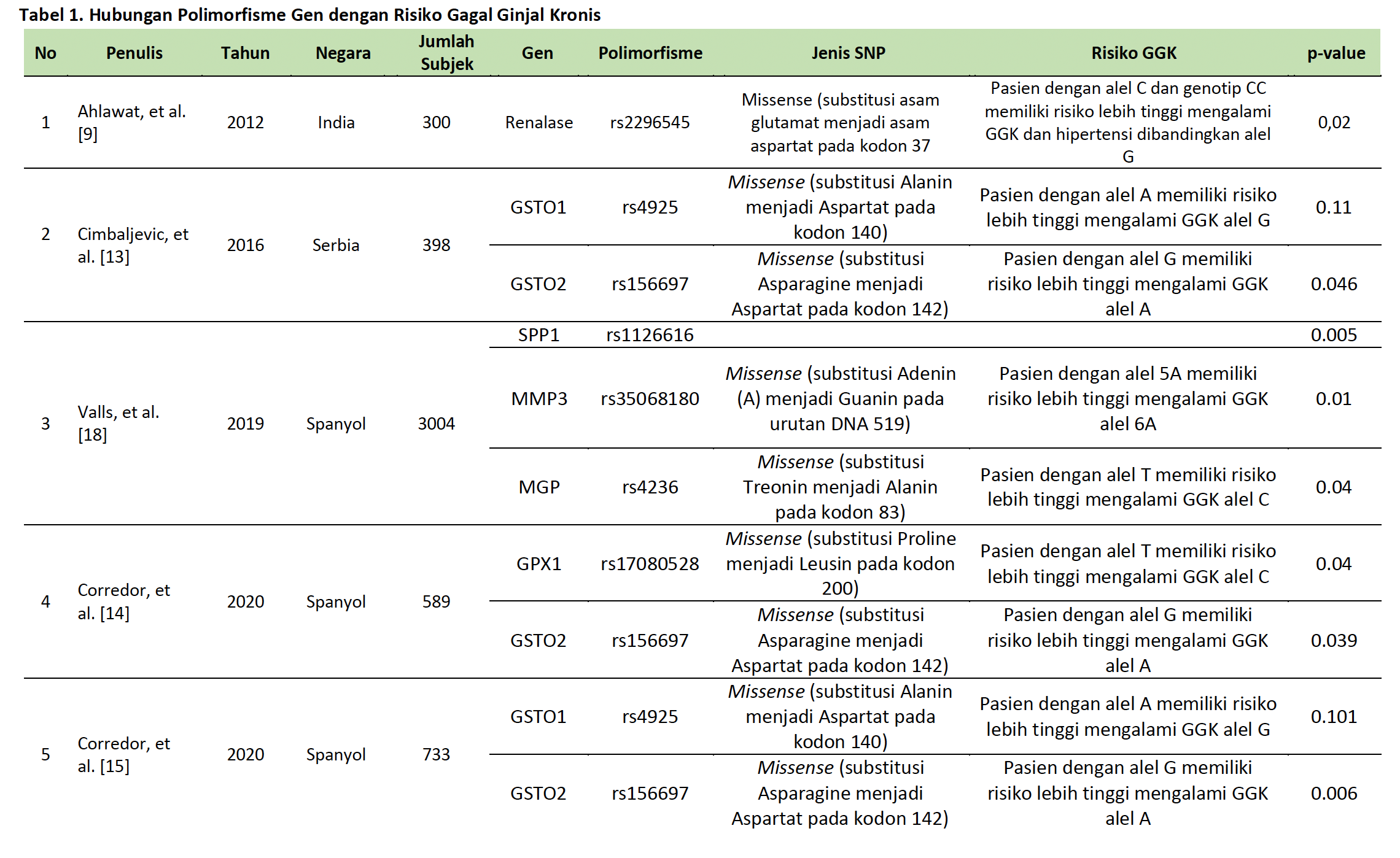
Gagal ginjal kronis (GGK) merupakan masalah kesehatan global yang terus meningkat, khususnya di Indonesia. GGK ditandai oleh penurunan fungsi ginjal yang bersifat progresif dan ireversibel, dengan diabetes melitus dan hipertensi sebagai faktor risiko utama. Polimorfisme gen yaitu variasi dalam sekuens DNA yang mempengaruhi fungsi gen dan ekspresi protein, memainkan peran penting dalam risiko dan perkembangan GGK. Studi ini meninjau peran polimorfisme genetik terhadap risiko GGK berdasarkan literatur yang tersedia. Narrative review ini menelusuri artikel ilmiah dari database meliputi PubMed, ScienceDirect, Google Scholar, dan Scopus, dengan kata kunci terkait polimorfisme genetik dan GGK. Dari 441 artikel yang ditemukan, seleksi dilakukan berdasarkan relevansi, jumlah subjek, dan kelengkapan artikel. Beberapa bentuk polimorfisme gen menunjukkan adanya hubungan yang signifikan terhadap peningkatan risiko gagal ginjal kronis, termasuk Renalase (rs2296545), GSTO1 (rs2164624), GSTO2, MMP3 (rs35068180), dan MGP (rs4236), CYP24A1, GPX1, UMOD, CYP2C8, CYP4A11, EPHX2, SPP1, dan BGLAP. Polimorfisme ini mempengaruhi mekanisme seperti regulasi tekanan darah, stres oksidatif, inflamasi, dan kalsifikasi jaringan, yang semuanya berkontribusi terhadap perkembangan GGK.
Disimpulkan bahwa polimorfisme genetik memainkan peran penting dalam risiko GGK, memberikan wawasan untuk pendekatan medis yang lebih personal dalam mendiagnosis, mencegah, dan merawat kondisi ini. Temuan ini mendukung pengembangan strategi pengobatan berbasis genetik yang lebih efektif di masa depan.
Referensi
[1] Kementrian Kesehatan Republik Indonesia. Laporan Nasional: RISKESDAS 2018. Jakarta, Indonesia: Badan Penelitian dan Pengembangan Kesehatan; 2019.
[2] Dipiro JT, Yee GC, Posey LM, Haines ST, Nolin TD, Ellingrod V. Pharmacotherapy: A Pathophysiologic Approach. 11th ed. McGraw Hill Medical; 2020.
[3] Menteri Kesehatan Republik Indonesia. Pedoman Nasional Pelayanan Kedokteran: Tata Laksana Penyakit Ginjal Kronik 2023.
[4] Hewagama A, Richardson B. The genetics and epigenetics of autoimmune diseases. Journal of Autoimmunity 2009;33:3–11. https://doi.org/10.1016/j.jaut.2009.03.007.
[5] Wang Y, He W. Endogenous Mitochondrial Aldehyde Dehydrogenase-2 as an Antioxidant in Liver. The Liver, Elsevier; 2018, p. 247–59. https://doi.org/10.1016/B978-0-12-803951-9.00021-5.
[6] International Consortium for Blood Pressure Genome-Wide Association Studies, Ehret GB, Munroe PB, Rice KM, Bochud M, Johnson AD, et al. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature 2011;478:103–9. https://doi.org/10.1038/nature10405.
[7] Al-Ajeeli ZA, Hameed HH. The Renalase (rs2296545) Polymorphism is Associated with End-Stage Renal Disease 2024;7:16–20.
[8] Malyszko J, Bachorzewska-Gajewska H, Dobrzycki S. Renalase, kidney and cardiovascular disease: are they related or just coincidentally associated? Adv Med Sci 2015;60:41–9. https://doi.org/10.1016/j.advms.2014.10.001.
[9] Ahlawat RS, Gupta S, Kapoor S, Kar P. Polymorphism of Renalase Gene in Patients of Chronic Kidney Disease. OJNeph 2012;02:136–43. https://doi.org/10.4236/ojneph.2012.24021.
[10] Khater MH, Abd EL-Hassib DM, Sabry JH, Elkilany RM, Ameen SG. Association Between Renalase Gene Polymorphism (rs2296545) and Hypertension in Egyptian Chronic Kidney Disease Patients. Cureus 2023. https://doi.org/10.7759/cureus.47903.
[11] Lv Y-B, Wang Y, Ma W-G, Yan D-Y, Zheng W-L, Chu C, et al. Association of Renalase SNPs rs2296545 and rs2576178 with the Risk of Hypertension: A Meta-Analysis. PLOS ONE 2016;11:e0158880. https://doi.org/10.1371/journal.pone.0158880.
[12] Board PG, Menon D. Structure, function and disease relevance of Omega-class glutathione transferases. Arch Toxicol 2016;90:1049–67. https://doi.org/10.1007/s00204-016-1691-1.
[13] Cimbaljevic S, Suvakov S, Matic M, Pljesa-Ercegovac M, Pekmezovic T, Radic T, et al. Association of GSTO1 and GSTO2 Polymorphism with Risk of End-Stage Renal Disease Development and Patient Survival. Journal of Medical Biochemistry 2016;35:302–11. https://doi.org/10.1515/jomb-2016-0009.
[14] Corredor Z, Da Silva Filho MI, Rodríguez-Ribera L, Catalano C, Hemminki K, Coll E, et al. Loci associated with genomic damage levels in chronic kidney disease patients and controls. Mutation Research/Genetic Toxicology and Environmental Mutagenesis 2020;852:503167. https://doi.org/10.1016/j.mrgentox.2020.503167.
[15] Corredor Z, Filho MIDS, Rodríguez-Ribera L, Velázquez A, Hernández A, Catalano C, et al. Genetic Variants Associated with Chronic Kidney Disease in a Spanish Population. Sci Rep 2020;10:144. https://doi.org/10.1038/s41598-019-56695-2.
[16] Wan J, Zhang G, Li X, Qiu X, Ouyang J, Dai J, et al. Matrix Metalloproteinase 3: A Promoting and Destabilizing Factor in the Pathogenesis of Disease and Cell Differentiation. Front Physiol 2021;12. https://doi.org/10.3389/fphys.2021.663978.
[17] Yamada. Association of gene polymorphisms with chronic kidney disease in high- or low-risk subjects defined by conventional risk factors. Int J Mol Med 2009;23. https://doi.org/10.3892/ijmm_00000193.
[18] Valls J, Cambray S, Pérez-Guallar C, Bozic M, Bermúdez-López M, Fernández E, et al. Association of Candidate Gene Polymorphisms With Chronic Kidney Disease: Results of a Case-Control Analysis in the Nefrona Cohort. Front Genet 2019;10:118. https://doi.org/10.3389/fgene.2019.00118.
[19] Darraji M, Saqban L, Mutar T, Rasheed M, Hussein A. Association of Candidate Genes Polymorphisms in Iraqi Patients with Chronic Kidney Disease. J Adv Biotechnol Exp Ther 2022;6:687. https://doi.org/10.5455/jabet.2022.d147.
[20] Jerotic D, Matic M, Suvakov S, Vucicevic K, Damjanovic T, Savic-Radojevic A, et al. Association of Nrf2, SOD2 and GPX1 Polymorphisms with Biomarkers of Oxidative Distress and Survival in End-Stage Renal Disease Patients. Toxins 2019;11:431. https://doi.org/10.3390/toxins11070431.
[21] Suárez-Santisteban MA, Santos-Díaz G, García-Bernalt V, Pérez-Pico AM, Mingorance E, Mayordomo R, et al. Association between CYP4A11 and EPHX2 genetic polymorphisms and chronic kidney disease progression in hypertensive patients. Nefrología (English Edition) 2024;44:382–95. https://doi.org/10.1016/j.nefroe.2024.01.020
Unduhan
Diterbitkan
Terbitan
Bagian
Lisensi
Hak Cipta (c) 2024 Penulis

Artikel ini berlisensi Creative Commons Attribution-NonCommercial 4.0 International License.




