In-use Stability of Hydrocortisone Injection Preparations at a Regional Referral Hospital in Central Java
DOI:
https://doi.org/10.36733/medicamento.v11i2.11538Keywords:
hospital, hydrocortisone, injection, in-use stabilityAbstract
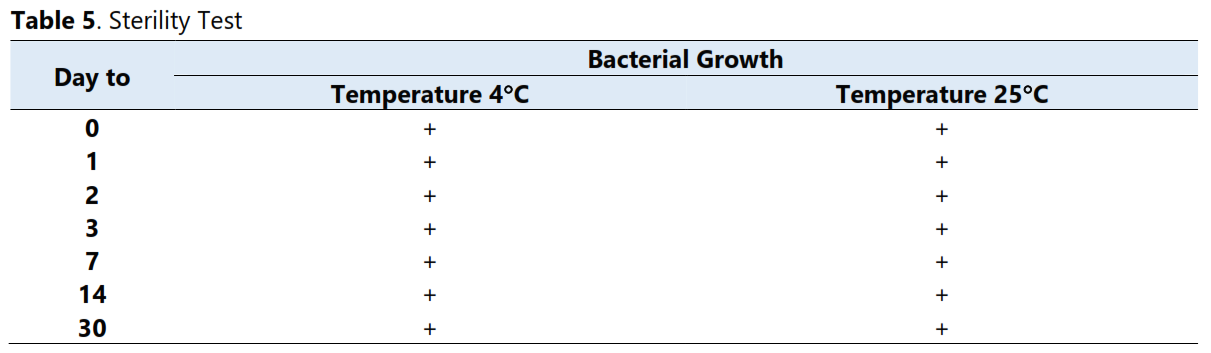
Hydrocortisone Sodium Succinate 50mg injection is widely used in perinatology wards. These wards cater to newborns (0-28 days), low birth weight (<2.5 kg), or premature babies (<37 weeks), requiring special handling. The use of this injection requires a very small dose, so one vial of hydrocortisone injection can be used for more than one patient. Therefore, the remainder of this hydrocortisone injection is often stored for 24 hours in the refrigerator which will later be reused on other patients. This study aims to determine the in-use stability of hydrocortisone injection stored for 24 hours at 4oC and 25oC. This is certainly to improve patient safety. The data collection technique uses an observational method on hydrocortisone injection samples with 3 replications. In-use stability is assessed from the results of organoleptic tests, pH tests, viscosity tests, determination of drug levels using UV-VIS spectrophotometry and sterility tests. Testing was conducted on days 0, 1, 2, 3, 7, 14 and 30. The results of the study showed that hydrocortisone injection preparations stored for 1 day at 4°C or 25°C in the perinatology ward were no longer physicochemically stable (concentration). Chemical degradation began on day 1, then microbial contamination occurred immediately after completion of compounding in the ward (day 0). This is because reconstitution was not carried out in a clean room in accordance with USP <797>. It can be concluded that hydrocortisone injection preparations prepared in this hospital are not recommended for administration to patients after 1 day of storage and must be compounded in a clean room.
References
1. Benjamin, Gayer G, Gayer S, Grooms S, Holcombe B. Pharmaceutical Compounding Roundtable: How Can Outsourcing Facilities Meet Provider Needs. American Society of Health-System Pharmacist; 2016.
2. Dewi SS, Rahmawati F, Pratiwi SUT. Kontaminasi Bakteri pada Sediaan Campuran Intravena di Bangsal Perawatan Rumah Sakit. J. Sains Farm. Klin. 2018;5(1):7-11.
3. Melviya, Yuliani SH, Putri DCA. Evaluasi Peracikan Sediaan Steril untuk Pasien Pediatri Rawat Inap di Rumah Sakit “ X ” Kota Semarang , Indonesia. J Manaj Dan Pelayanan Farm. 2019;8(3):128-135.
4. Suvikas-peltonen E, Hakoinen S, Celikkayalar E, Laaksonen R, Airaksinen M. Incorrect aseptic techniques in medicine preparation and recommendations for safer practices : a systematic review. Eur. J. Hosp. Pharm. 2017:175-181. doi:10.1136/ejhpharm-2016-001015
5. Legeay C, Bourigault C, Lepelletier D, Zahar JR. Prevention of Healthcare-associated Infections in Neonates: Room for Improvement. J Hosp Infect. 2015;89(4):319-323. doi:10.1016/j.jhin.2015.02.003
6. Ramasethu J. Prevention and Treatment of Neonatal Nosocomial Infections. Matern Health Neonatol Perinatol. 2017;3(1):5. doi:10.1186/s40748-017-0043-3
7. USP. General Chapter <797> Pharmaceutical Compounding – Sterile Preparations. In: USP 31. Pharmacopeial Convention; 2023:318. https://www.usp.org/events-training/course/usp-general-chapter-797-pharmaceutical-compounding-sterile-preparations
8. Depkes RI. Pedoman Dasar Dispensing Sediaan Steril. Departemen Kesehatan Republik Indonesia; 2009.
9. Genatrika E. Pedoman Dasar Penyiapan Produk Steril Non Sitostatika. UMP Press; 2023.
10. Rambe R, Gultom ED, Zulfikri Z, Rani Z, Susanti E, Athaillah A. Evaluasi Dispensing Sediaan Steril Antibiotik Pada Pasien Pediatri Di Rumah Sakit X. Forte J. 2023;3(2):167-176. doi:10.51771/fj.v3i2.636
11. Genatrika E, Puspitasari I, Kristina SA, Sulaiman TNS. Suitability in compounding sterile preparations: An observational study in a referral hospital. J Pharm Pharmacogn Res. 2022;10(2):338-348. doi:10.56499/jppres21.1305_10.2.338
12. Putri DCA, Yuliani SH. Evaluasi Peracikan Injeksi Seftriakson di Salah Satu Rumah Sakit Swasta di Semarang. J Farm Klin Indones. 2018;7(3):143-153.
13. Lee JH, Moriyama B, Henning SA, et al. Stability of isoniazid injection in i.v. solutions. Am J Health-Syst Pharm AJHP Off J Am Soc Health-Syst Pharm. 2018;75(10):622-626. doi:10.2146/ajhp170268
14. Chen P, Chen F, Zhou BH. Compatibility and stability of dezocine and tropisetron in 0.9% sodium chloride injection for patient-controlled analgesia administration. Medicine (Baltimore). 2018;97(50):e13698. doi:10.1097/MD.0000000000013698
15. Yusefa IM, Harmastuti N, Harjanti R. Pengaruh Suhu Penyimpanan terhadap Kadar Parasetamol Sirup selama Beyond Use Date secara Spektrofotometri UV-Vis. Indones J Pharm Nat Prod. 2024;7(2):141-150.
16. Kemenkes RI. Farmakope Indonesia. Edisi VI. Kementerian Kesehatan Republik Indonesia; 2020.
17. Sagitha IGE, Suharjono, Yulistiani, Isnaeni. Uji Stabilitas Sediaan Ampisillin Sulbaktam setelah Rekonstitusi. Pharma Xplore J Sains Dan Ilmu Farm. 2023;8(1).
18. Berteau C, Filipe-Santos O, Wang T, Rojas HE, Granger C, Schwarzenbach F. Evaluation of the impact of viscosity, injection volume, and injection flow rate on subcutaneous injection tolerance. Med Devices Auckl NZ. 2015;8:473-484. doi:10.2147/MDER.S91019
19. Ko E, Song YJ, Choe K, Park Y, Yang S, Lim CH. The Effects of Intravenous Fluid Viscosity on the Accuracy of Intravenous Infusion Flow Regulators. J Korean Med Sci. 2022;37(9):1-5. doi:https://doi.org/10.3346/jkms.2022.37.e71
20. Prisma A, Djoko DJ, Masruroh. Pengaruh Konsentrasi dan Viskositas Larutan Polistiren terhadap Morfologi Permukaan dan Ketebalan Lapisan ZnPc Pada Permukaan QCM. Brawijaya Phys Stud J. Published online 2014:1-4.
21. Damayanti DAT. Rancangan Formulasi dan Teknologi Sediaan Steril Injeksi Fenitoin serta Uji Evaluatif Sediaan. J Kesehat Tambusai. 2024;5(2). Accessed December 26, 2024. https://journal.universitaspahlawan.ac.id/index.php/jkt/article/view/27349
22. Leanpolchareanchai J, Dilokpattanamongkol P, Lertwattanachai T, Trisataya A, Wongrakpanich A, Pravitharangul T. Stability of Hydrocortisone Sodium Succinate in Intensive Care Units: Focus on Practical Points. J Med Assoc Thai. 2022;105(4):321-326. doi:10.35755/jmedassocthai.2022.04.13293
23. Rashati D. Pengaruh Variasi Suhu Penyimpanan terhadap Stabilitas Fisik Suspensi Amoxicillin. J Ilm Farm AKFAR. 2017;2(2):27-32.
24. Almadani Alforjany E, Mohamed Kamour R. Effect of Temperature of Water Used for Reconstitution on Stability of Antibiotic Dry Suspension. J Med Chem Sci. 2019;2(4):177-183. doi:10.26655/jmchemsci.2019.8.8
25. Abiya FM, Ulfa M, Setyonnugroho W. Infection Control Risk Assessment (ICRA) di Instalasi Gawat Darurat Rumah Sakit PKU Muhammadiyah Gamping. Universitas Muhammadiyah Yogyakarta; 2017.
26. Madjid T, Wibowo, Adik. Analisis Penerapan Program Pencegahan dan Pengendalian Infeksi di Ruang Rawat Inap RSUD Tebet Tahun 2017. J ARSI. Published online 2017:57-68.
27. Nogler-Semenitz E, Lass-Flörl C, Nogler M, Speer G, Dierich MP. Bacterial contamination of solutions for parenteral administration for single- and multiple-dose vials after multiple use in the hospital. Wien Med Wochenschr 1946. 2007;157(15-16):398-401. doi:10.1007/s10354-007-0423-9
Downloads
Submitted
Accepted
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Erza Genatrika, Elza Sundhani, Adriana Eka Fitri

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.





















